Be Wary of Controlled and Non-Controlled Medication Replicas Sold Online
Problem: A pharmacy technician was shopping online and purchased a plastic drinking cup that resembled a propofol vial (Figure 1). She brought the cup into the pharmacy to use as a decoration. The pharmacy director was concerned to see this type of product, so took a closer look. It turns out, the label included a national drug code (NDC) and barcode. The pharmacy director then attempted to scan the barcode into an electronic health record (EHR) and, shockingly, found that it actually scanned and documented propofol!
We found that several similar products are available for purchase on the Etsy and Amazon websites, including drinkware designed to look like controlled substance medication vials such as fentaNYL, HYDROmorphone, ketamine, LORazepam, and midazolam. There were ornaments, candles, and even sippy cups (Figure 2 and Figure 3). While some items appear to be crafts using copies of drug labels, the ornaments appear to be made from actual medication vials, or nearly identical replicas. In fact, one propofol ornament even has a plastic hanger attached!

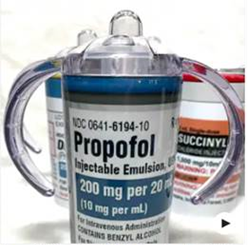
Not only do these replica products look like the real drug, but it is alarming when practitioners can scan the barcode on a drinking cup and document the administration of a medication on the medication administration record (MAR). With controlled substances, this could also be a potential diversion issue. For example, a practitioner could scan the barcode on a replica product (e.g., a drinking cup stored in the patient care unit) to falsely document in the MAR that a medication had been scanned and administered to a patient, and then keep the drug for personal use or distribution.
Rarely do you see something promote the “drug culture” as this does. In fact, it is rather disturbing how these products seem to be making light of the dangers of these drugs.
Mix-ups with promotional or demonstration (demo) products
We have previously shared that manufacturers sometimes promote medications with items that look like the drug product itself but serve another purpose, which creates a potential for errors. For example, we have several reports of confusion between supposed medical products that were actually promotional materials, including candies and body lotions. One case (February 9, 2006 newsletter) involved a dermatologist who gave a patient what he thought was a sample tube of a company’s ointment, but it was a magic marker that looked like the ointment container. A young woman then applied the “ointment” (actually purple ink) all over her face for treatment of facial contact dermatitis/eczema. She was seen later in an emergency department to treat a local reaction to the ink. In another case, when GLIADEL (carmustine implant) was first marketed, we received complaints about a marketing campaign in which chocolate candy was packaged to look like the real Gliadel wafer, which is implanted intracranially to treat malignant glioma.
Errors involving demonstration (demo) products have also been reported. For example, in 2015, ISMP and the US Food and Drug Administration (FDA) alerted healthcare professionals not to use Wallcur simulated (demo) intravenous (IV) products in human or animal patients. More than 40 patients received these nonsterile products that were distributed to medical clinics, surgical centers, and urgent care facilities intended for medical training purposes only. Some patients developed chills and/or sepsis. One patient died.
Given that patients and healthcare practitioners could be confused by online merchandise that looks like real medications, these types of products should not be permitted in healthcare organizations.
Safe Practice Recommendations: We have notified the US Food and Drug Administration (FDA) of this issue and recommend that non-drug products should not be allowed to have active NDCs and scannable barcodes. Organizations should be aware of the potential for drug diversion and the risk of patient harm if these products are mistaken or scanned surreptitiously in place of real drugs. Please consider the following recommendations.
Set expectations. Prohibit merchandise products that resemble actual medications (especially those with NDCs and barcodes) from being brought into or stored in the pharmacy or on patient care units. Review recommendations in our previous newsletter, Managing visits from pharmaceutical sales representatives, to ban these types of products from being brought into the organization and distributed to staff.
Do not reuse drug vials. Used or unused medication vials should never be removed from organizations for the purpose of using them as crafts or to be sold as merchandise.
Educate staff. Help staff understand the risk of purchasing these products online and bringing them to work. Discourage staff from making or selling these products outside of work. As healthcare practitioners knowledgeable about the risks, we should not be promoting or making light of the use of controlled substances in this manner.
Educate patients. If patients bring in replica drug products, inform them that your organization does not allow the use of these products due to the concern of mix-ups with real medications.
Reinforce. Consider incorporating this issue into the checklist when pharmacists and pharmacy technicians perform monthly unit inspections. Share concerns with nurse managers, physician leadership, Pharmacy & Therapeutics committee, and supply staff. If replica drug products are found, coach staff about the risks and the organization's policies.
Suggested citation:
Institute for Safe Medication Practices (ISMP). Be wary of controlled and non-controlled medication replicas sold online. ISMP Medication Safety Alert! Acute Care. 2024;29(2):1-3.
Access this Free Resource
You must be logged in to view and download this document.
