Patient Death Tied to Lack of Proper Escalation Process for Barcode Scanning Failures
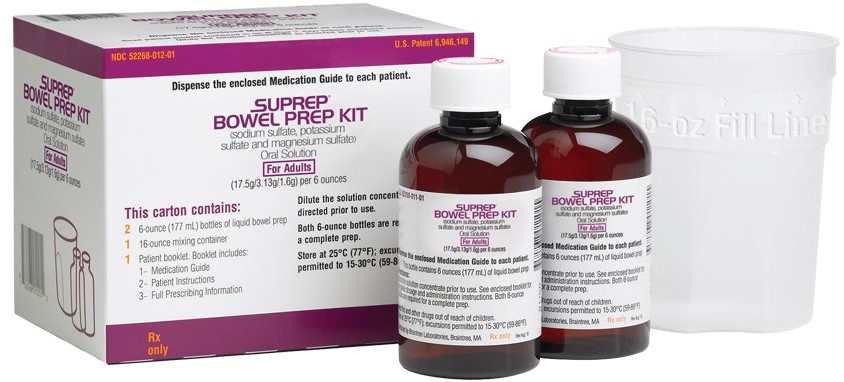
Problem: A patient who was hospitalized in the intensive care unit (ICU) for rectal bleeding was scheduled to have a colonoscopy the following day. A prescriber ordered SUPREP BOWEL PREP KIT (sodium sulfate, potassium sulfate, and magnesium sulfate) (Figure 1) to be administered orally for cleansing of the colon as a preparation for the colonoscopy. Unfortunately, instead of Suprep, the patient was mistakenly given NATURALYTE, which is a liquid acid concentrate for bicarbonate hemodialysis, used as a dialysate with hemodialysis equipment after proper dilution. The patient later died and local media covered the incident. Via an open records request to the state board of nursing that investigated the situation given a nurse’s involvement in the error, ISMP obtained a report that helped to detail system failures that contributed to this tragic medication error.
NaturaLyte, which is available in a large plastic container, had been left in the ICU by the dialysis team for a different patient who was undergoing hemodialysis about 3 days before this incident. The large container was placed in the same medication area as are other bulk items when delivered from pharmacy. When it was time to administer the bowel prep, the nurse went to the medication area and saw two large plastic containers labeled NaturaLyte, containing a clear liquid. The nurse assumed these were similar to GOLYTELY (polyethylene glycol 3350 and electrolytes for oral solution), which is widely used as a bowel prep and apparently more familiar than Suprep. The board report voiced a concern that the NaturaLyte label was not visually double-checked before giving it to the patient in error. However, this may not have raised a red flag if the nurse thought NaturaLyte was a generic replacement for GoLYTELY, given that many generic products have different brand names than the original product name. In addition, NaturaLyte and GoLYTELY show similarities, namely the NaturaLyte label lists ingredients including magnesium, potassium, and sodium, in the same manner as the container of GoLYTELY lists electrolytes. Also, both are in large plastic containers (Figure 2). The board report did not mention whether the actual Suprep product had been dispensed by the pharmacy and was present on the unit but not located by the nurse.
Although NaturaLyte has a barcode, the barcode may not be recognized by many barcode scanning systems since NaturaLyte is not a drug and the barcode does not contain a national drug code (NDC). In this case, the nurse did try to scan the item several times, but when the misidentified product could not be successfully scanned, the nurse called pharmacy before proceeding. Rather than sending a new labeled medication (Suprep), or physically reviewing the product that would not scan, a pharmacist sent a patient label that contained a barcode through the tube system for the correct medication, Suprep.
When used properly as dialysate, NaturaLyte must first be diluted in a 1:44 ratio with purified water and base concentrate (bicarbonate) before it can be instilled. However, thinking that the NaturaLyte product was the same as GoLYTELY (and assuming that was substituted for Suprep), the nurse scanned the patient’s armband, scanned the label provided by pharmacy, and administered about 240 mL of the NaturaLyte in its concentrated form. The patient began to drink the liquid but could not tolerate it all due to the bad taste and became nauseous.
Since the entire amount of product was supposed to be administered for the prep, and since the patient could not tolerate it and refused to drink the liquid, the nurse notified the physician. The physician noted that a feeding tube would be needed to administer the remainder of the medication. Another nurse (on the next shift) administered the rest of the concentrated NaturaLyte liquid through the feeding tube. The second nurse also thought that Suprep was similar to GoLYTELY, and was substituted with NaturaLyte. A physician who later assessed the condition as the patient deteriorated, also thought the container looked like GoLYTELY. Later, an electrocardiogram (EKG) revealed significant changes and the patient died the following morning. The cause of death was not mentioned in the report.
It should be noted that on the day the error happened, some ICU nurses were pulled to other hospital areas, leaving the nurse involved with this patient caring for two other high acuity patients as well, rather than the typical assignment of just two patients total. Also, we do not know if staffing levels in the pharmacy may have impacted the pharmacist’s ability to visually confirm the product. In addition, since the product was a large plastic container, this may have prevented it from being tubed back to the pharmacy for visual confirmation.
ISMP has previously received reports from other hospitals in which dialysis products were left in patient care areas where staff may be unfamiliar with or do not know about their proper use. For example, we have previously published a report involving 23.4% sodium chloride injection vials left on nursing units by hospital-contracted dialysis staff while providing treatment to inpatients using portable hemodialysis machines. Hypertonic sodium chloride injection is sometimes used to reduce cramping during hemodialysis. However, the vials have been confused with sodium chloride 0.9% by staff unfamiliar with the highly concentrated product.
We also know that it is not only dialysis staff that might leave items that are unfamiliar to others on nursing units. We wrote about similar events, first in 2005, then in 2010, in which a transplant team left behind a bag of VIASPAN cold storage solution used in organ transplantation, which ended up in a pharmacy return bin because it looked so much like an intravenous (IV) solution bag. Inadvertent IV administration of the solution would almost certainly cause cardiac arrest due to the high potassium content (about 125 mEq/L). We have also received reports where providers have brought in nonformulary medications for their patients.
Safe Practice Recommendations: Serious medication errors often involve unfamiliar products, as happened in the current case. Therefore, it is important to have processes in place to prevent these types of errors. For non-unit dose products, rather than include a pharmacy-generated barcode on the medication label, practitioners should scan the manufacturer barcode directly on the product. This type of forcing function ensures the right container is in hand to prevent the risk of a false positive barcode scan from just a pharmacy or patient label. Also, develop an escalation process for what to do when a medication barcode will not scan. When a barcode will not scan, pharmacists need to visually verify that the medication matches what is ordered for the patient. It is not safe to send a label by itself. Labels must be considered part of the dispensing process and should only be placed on products by pharmacy personnel.
In addition, Suprep is not available in a large plastic container. Instead, bottles of Suprep must be further diluted and patients must drink with additional water. Given that staff were apparently unfamiliar with the product versus GoLYTELY, it points out the need for widespread inservice education, memos, internal newsletter articles, and/or huddles when new products are being introduced to the formulary and when they are being dispensed to areas of the hospital where they are not normally used. When performing monthly unit inspections, pharmacists and pharmacy technicians should notify pharmacy and unit leaders of products that are found in patient care areas that do not belong there and remove them immediately. When outside groups contract to provide services, hospital leadership must notify the pharmacy director to ensure that the medications and dosage forms that might be used are reviewed and agreed upon by the Pharmacy and Therapeutics Committee. At that time, alternative products may be discussed and/or arrangements made to securely store products normally unavailable at the hospital.
Suggested citation:
Institute for Safe Medication Practices (ISMP). Patient death tied to lack of proper escalation process for barcode scanning failures. ISMP Medication Safety Alert! Acute Care. 2023;28(19):1-3.
Access this Free Resource
You must be logged in to view and download this document.
