Implement Strategies to Prevent Persistent Medication Errors and Hazards: 2024
Reflecting on events that occurred in 2023, we have identified the top three medication errors and hazards that were recurring themes in the ISMP Medication Safety Alert!, which our affiliate ECRI, included in ECRI’s Top 10 Patient Safety Concerns for 2024. Our selected top concerns are not solely based on the most frequently reported problems or those that have led to the most serious consequences for patients, although these factors were considered. Rather, we focused on errors and hazards that continue to occur but can be avoided or minimized with system and/or practice changes. If you have not already taken action to mitigate these risks, we hope awareness of these informs the priorities you set for your medication safety improvement plan!
Lack of Proper Escalation Process for BCMA Scanning Failures
Problem: Barcode medication administration (BCMA) systems are valuable tools that reduce medication administration errors, but only when used correctly. Staff must know how to properly use the system, and procedures must include an escalation process for barcode scanning failures. Otherwise, practitioners may employ workarounds when a barcode is hidden or damaged; is difficult to scan (e.g., white print on clear bag); is missing; or when a medication has not yet been added to the computer system. BCMA workarounds may indicate that the devices and systems are not configured to support safe clinical workflow, that the staff have received insufficient education related to appropriate BCMA use, or they lack knowledge about the risks involved when employing a workaround.
Examples of unsafe BCMA practices include administering a medication even though the barcode will not scan, scanning after medication administration, or scanning barcodes from sources other than the medication itself (i.e., proxy scanning), such as scanning the barcode on an already hanging empty bag or a barcode not affixed to the product actually being administered. In our September 21, 2023 newsletter article, Patient death tied to lack of proper escalation process for barcode scanning failures, we shared how a prescriber ordered SUPREP BOWEL PREP KIT (sodium sulfate, potassium sulfate, magnesium sulfate) to be administered orally for a patient with rectal bleeding in preparation for a colonoscopy. Unfortunately, instead of Suprep, the nurse mistakenly administered NATURALYTE, which is a liquid acid concentrate that is diluted and then used as a dialysate with hemodialysis equipment.
Like many preventable adverse drug events, there were several system failures that contributed to the Suprep incident, including the lack of an escalation process when a barcode would not scan. Prior to administering what was thought to be Suprep, the nurse tried to scan the barcode on the NaturaLyte container several times. When the misidentified product could not be successfully scanned, the nurse called the pharmacy. Rather than sending a new labeled Suprep container, or physically reviewing the product that would not scan, a pharmacist sent a patient label that contained a barcode for the correct medication, Suprep, so the nurse could apply it to the container on hand (NaturaLyte). The nurse scanned the patient’s armband, scanned the label provided by pharmacy, and administered the NaturaLyte. Later, an electrocardiogram (EKG) revealed significant changes and the patient died the following morning.
Recommendations: Have the medication safety committee review practices that lead to BCMA workarounds and develop modifications and configurations that address system issues and support safe clinical workflow. Test new product barcodes in the pharmacy prior to distribution. When available, practitioners should scan the manufacturer’s barcode printed directly on the product. This ensures the right (or wrong in the above case) container is in hand to prevent the risk of a false positive barcode scan from just a pharmacy-applied label.
If a medication barcode will not scan, the practitioner must review the patient’s medication administration record (MAR) to confirm if it is the intended product for the right patient at the right time (e.g., ensure the order has not been modified or discontinued). Develop an escalation process when a medication barcode will not scan. The process should include when and how to report barcode-related issues, why it is dangerous to use a proxy scan, and who is responsible for monitoring barcode issues. When a barcode will not scan, pharmacists need to visually verify that the medication matches what is ordered for the patient. It is not safe to send a label by itself. Labels must be considered part of the dispensing process and should only be applied by pharmacy personnel on pharmacy-prepared products.
Regularly review BCMA data to identify medications commonly administered without scanning to help identify potential workflow or product issues. Educate end users to report barcode issues so that pharmacy can assess for contributing factors related to workarounds or equipment malfunctions, and consider an alternative product, when possible. Use internal or external published events related to incorrect BCMA utilization to educate staff to further highlight the importance of BCMA. Report repetitive product scanning issues to ISMP so we can work with manufacturers, and report issues to the US Food and Drug Administration (FDA), to improve the safety of product labeling and packaging.
Misuse of Parenteral Syringes to Administer Oral Liquid Medications
Problem: Using parenteral syringes—syringes with Luer connectors that can mistakenly be attached to needleless intravenous (IV) systems—allows for improper administration of oral/enteral liquid medications IV, which is a significant patient safety risk. The unintended administration of oral liquid medications via the IV route can result in serious patient harm, including infection and/or pulmonary emboli, and even death. The risk of misadministration can be reduced or eliminated through consistent use of oral or enteral (ENFit) syringes for preparation and administration of oral/enteral liquids. These syringes have specially engineered hubs that cannot be easily or securely attached to standard Luer connectors.
However, inexperienced nurses and other providers may not be aware of the differences between oral, enteral, and parenteral syringes (Figure 1) and the associated risks. Sadly, we continue to receive reports in which patients were inadvertently given an oral liquid medication intravenously. For example, a nurse withdrew a dose of oral oxyCODONE liquid from a unit dose cup into a parenteral syringe, as no oral or enteral syringes were available. The nurse inadvertently attached the syringe to an IV access port and injected it. Also, fatalities have occurred when the contents of liquid-filled capsules (e.g., niMODipine) were drawn into a parenteral syringe (because a needle was needed to withdraw the medications), intending to be administered via an enteral tube but were inadvertently administered IV.
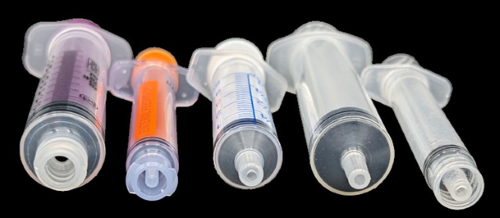
Recommendations: The ISMP Targeted Medication Safety Best Practices for Hospitals Best Practice #4 (archived in 2022) calls for organizations to ensure that all oral liquid medications that are not commercially available in unit dose packaging are dispensed by the pharmacy in an oral syringe, or else an enteral syringe that meets the International Organization for Standardization (ISO) 80369 standard, such as ENFit. Organizations should purchase, store, and maintain an adequate supply of oral/ENFit syringes in all patient care areas wherever oral/enteral medications are prepared and administered, and the pharmacy should verify, with every monthly unit inspection, that these are in stock and readily available. During hospital consulting engagements, even in hospitals that are committed to stocking oral/ENFit syringes, we find that they are not consistently available where needed.
It is important that staff know why parenteral syringes should never be used to prepare or administer oral liquid medications, so this should be included as part of orientation and then reinforced on a regular basis. ISMP has identified that despite regular mention of this issue, this information is not routinely included as part of the academic curricula for healthcare practitioners, nor does this safety issue appear in fundamental textbooks used by many academic settings, so orientation may be the first time a new practitioner hears about this risk. Take every opportunity to communicate the rationale behind using ENFit syringes as a forcing function to prevent wrong-route misconnections. If not already done, leaders should make it an organizational priority to convert to ENFit enteral devices as soon as practical. Refer to the Global Enteral Supplier Device Association’s (GEDSA’s) ENFit Pharmacy Resource Guide for more information.
Drug, Supply, and Equipment Shortages Continue to Compromise Patient Care
Problem: Drug, supply, and equipment shortages disrupt the ability to meet patient needs across the care continuum, often causing delayed treatments and services, poor patient outcomes, and increased costs. Last year, ISMP and ECRI conducted a survey on Drug, Supply, and Equipment Shortages, and the results were shared in our September 7, 2023 newsletter. Nearly 200 respondents reported shortages affecting several clinical departments: surgery/anesthesia (74%), emergency care (64%), pain management (52%), cardiology (45%), hematology/oncology (44%), infectious disease (39%), and obstetrics/gynecology (37%)—some resulting in serious adverse effects.
Nearly one-third (32%) of the respondents were unable to provide patients with the recommended drug or treatment for their condition, and more than one-fifth (21%) thought this resulted in patients receiving a less effective drug. Adverse patient outcomes that were reported included interrupted, modified, or delayed chemotherapy regimens (e.g., reduced doses, treatment withheld if non-curative intent), and rescheduling, postponing, or canceling surgical cases due to lack of supplies needed for procedures.
Almost a quarter (24%) of the respondents were aware of at least one medical/medication error related to a drug, supply, or device shortage in the 6 months prior to the survey. Respondents provided descriptions of more than 40 errors. For example, a patient received 10 mg of oxyCODONE instead of 5 mg when a 10 mg tablet needed to be used during a shortage of 5 mg tablets. The oxyCODONE 10 mg tablet was intended to be split and only a half tablet was to be administered. In other examples, multi-dose vials of lidocaine and methotrexate were inadvertently used when preservative-free vials were required.
The impact of drug, supply, and equipment shortages continues to exert an enormous toll on healthcare providers and patients. A vast amount of effort is spent on planning for and managing shortages. This includes educating staff; stocking alternative products and adding barcodes into systems; dealing with secondary market vendors; prescribing, preparing, and administering unfamiliar alternative products; and fielding questions. All of this consumes a large portion of the health professionals’ time, stealing valuable resources from other activities.
Recommendations: Preparation, standardization, communication, and monitoring are paramount to safely managing drug, supply, and equipment shortages. Although it may be impractical to prepare for every potential shortage, proper planning can minimize the adverse effects for both patients and practitioners. Take routine inventory of all critical supplies and perform periodic risk assessments to identify and prioritize vulnerable items. Be sure to update and standardize any processes associated with alternative medications, supplies, or equipment. For more information, refer to ISMP newsletters, Weathering the storm: Managing the drug shortage crisis, A comprehensive, proactive plan is needed to mitigate risk when changing drug concentrations, and Safety considerations during expedited product approval. Use error and adverse event reporting systems as well as focus group meetings, discussions during rounds, or other means, to learn about hazardous conditions, close calls, and adverse events associated with shortages, to limit further risk and harm.
Multiple resources are available to help manage this complex problem, including the following:
- ASHP Shortage Resources webpage
- FDA Drug Shortages webpage
- FDA Medical Device Supply Chain and Shortages webpage
- US Department of Health and Human Services Medical Product Shortages and Scarce Resources webpage
- Centers for Disease Control and Prevention Current Vaccine Shortages & Delays webpage
- American Society for Parenteral and Enteral Nutrition (ASPEN) Parenteral Nutrition Resources webpage
- ECRI Supply Chain Disruption in Healthcare webpage
Suggested citation:
Institute for Safe Medication Practices (ISMP). Implement strategies to prevent persistent Medication errors and hazards: 2024. ISMP Medication Safety Alert! Acute Care. 2024;29(6):1-4.
Access this Free Resource
You must be logged in to view and download this document.
