Rabies Immune Globulin Vial Sizes Look Similar
A Safety Brief in the June 28, 2018, ISMP Medication Safety Alert! noted a change in concentration for rabies immune globulin (human) (HYPERRAB) manufactured by Grifols. Since a higher concentration allows for more efficient wound infiltration (i.e., more of the immune globulin can be delivered to the affected area in less volume), the concentration was increased from 150 units per mL to 300 units per mL. When stocking, storing, and dispensing HyperRAB, it is important to recognize that the product is available in both 1 mL and 5 mL vials and cartons that are hard to tell apart (Figure 1). The only visual differences are the volume noted in the lower left corner of the carton and different NDC numbers. The vials themselves both have a 5 mL capacity although one contains 5 mL while the other contains only 1 mL.
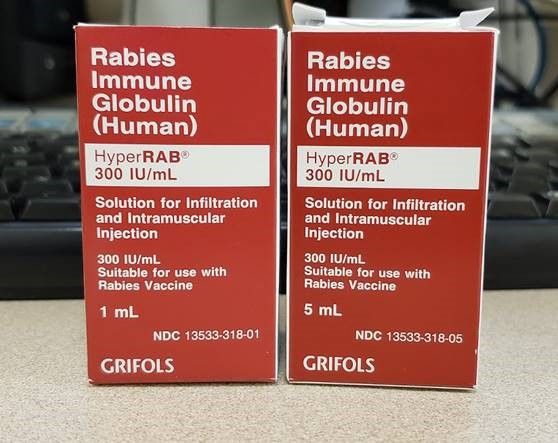
According to the package insert, the 1 mL vial is sufficient for a child weighing 15 kg, while the 5 mL vial is sufficient for an adult weighing 75 kg. Both are single-use vials. While each vial contains 300 units per mL, confusing the vial sizes may lead to costly waste if a 5 mL vial is dispensed and used for a child. The situation could also result in inventory issues. For example, someone may visually scan their inventory, not notice the volume differences, and mistakenly believe enough product is on hand when there may be far less than needed to treat patients. To prevent this, one hospital is storing the two sizes apart from one another along with a note to double check the vial volume before using. Auxiliary labels to better differentiate the products and the use of barcode scanning during inventory and product selection are additional risk-reduction strategies. ISMP has also forwarded complaints to the manufacturer and the US Food and Drug Administration (FDA).
