Container label changes for vitamin A, D, and E
You may have noticed changes in the units of measure on the labels of over-the-counter (OTC) fat-soluble vitamins (A, D, and E) from international units (IU) to metric units of measure—micrograms (mcg) or milligrams (mg). For example, aqueous vitamin D oral drops previously labeled as 400 international units per mL is now labeled as 10 mcg per mL (Figure 1). This change also involves OTC solid dosage forms, but it does not include prescription products such as AQUASOL A (water-miscible vitamin A palmitate). These changes are based on a US Food and Drug Administration (FDA) final rule, “Food Labeling: Revision of the Nutrition and Supplement Facts Labels,” published in the Federal Register on May 27, 2016 (page 33748). The rule also requires listing the absolute amounts of vitamins and minerals in mg or mcg in addition to the percent daily value (% DV) on the label.
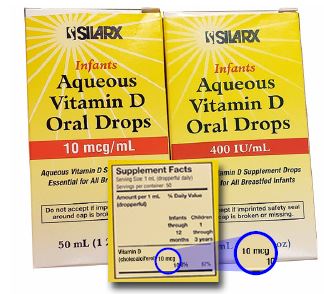
mcg and units should be listed.
Unfortunately, most healthcare practitioners and consumers are unaware of the change, and the labeling may not be helpful in communicating the change. Only the metric measure may appear on container labels, including the Supplement Facts label (Figure 1), making it difficult to identify the equivalency between the previous measure in international units and the new metric measure. Still, the notice in the Federal Register mentions that, “The amount of vitamin D may, but is not required to, be expressed in IUs [sic], in addition to the mandatory declaration in mcg. Any declaration of the amount of vitamin D in IUs [sic] must appear in parentheses after the declaration of the amount of vitamin D in mcg.” Note that IU is used here within quotation marks, as it appears in the notice. However, ISMP discourages the use of IU. In our October 18, 2000 newsletter, we mentioned multiple cases in which IU was mistaken as IV. Given that vitamins such as E are available in oily liquids, a wrong route error is possible. Such a mistake could prove harmful, even fatal.
ISMP fully supports including the strength on container labels and Supplement Facts panels in both mcg or mg as well as international units in parentheses to allow for safe transition to metric-only labeling. Incidentally, the labeling changes do not reflect changes in strength. One mcg of vitamin D (cholecalciferol) is equal to 40 international units, so 10 mcg is the same as 400 international units on the new label. We have been in touch with the FDA Center for Food Safety and Applied Nutrition (CFSAN) about this situation and encourage the agency to consider a public announcement about the change. Practitioners will most likely still recommend doses in units, and this will be confusing to patients. Thus, we also hope that manufacturers will express the units in parentheses after the metric strength.
