Weathering the Storm: Managing the Drug Shortage Crisis
All healthcare organizations have disaster plans in place that they practice and refine in preparation for an unexpected crisis. These plans are not developed “on the fly” because healthcare providers recognize the value of planning for the unexpected and the necessity of minimizing potentially life-saving interruptions in care. The ongoing problem with drug shortages in our nation is rising to the level of “disaster” status. Drug shortages continue to take an enormous toll on healthcare providers who must deal with the problem on a daily basis, and on patients who are on the receiving end of the shortages.
According to more than 1,800 respondents to our recent 2010 survey, the conditions associated with drug shortages during the past year have been the worst ever, with little hope for improvement in the near future. Respondents were most alarmed by:
- The ever-increasing volume of critically important medications in short supply
- The use of less desirable, unfamiliar alternative drugs—if available
- Errors and poor patient outcomes caused by absent or delayed treatment or preventable adverse drug events caused by the use of alternative drugs or dosage forms
- The lack of advanced warnings about impending shortages
- Precious clinical hours lost to time-consuming activities required to manage drug shortages.
Overall, survey respondents conveyed a real sense of crisis and are clearly looking for support to reduce the organizational burden and potential patient harm associated with drug shortages. As previously mentioned, ISMP and the American Society of Health-System Pharmacists (ASHP) believe a public meeting with FDA and key stakeholders is urgently needed to develop a strategic plan aimed at reducing the occurrence of drug shortages and managing them better when they occur. More effective FDA oversight of drug shortages, a comprehensive early warning system, and patient safety and clinical outcomes placed ahead of anyone’s profit margins are goals we hope to begin to explore at this meeting.
Meanwhile, healthcare organizations, guided by pharmacy leadership, can follow the recommendations below to help manage this complex problem. These recommendations are primarily culled from a prior article ISMP published on drug shortages,1 ongoing discussions with healthcare providers, and guidelines published by ASHP in 2009.2 Some of the recommendations denote well-known and currently followed steps; others may provide new ideas for managing the problem. Either way, the recommendations can serve as a tool to evaluate your current processes for managing drug shortages. Although it may be impractical to prepare for every potential drug shortage, proper planning can minimize the adverse effects on both patients and providers.
Identify drug shortages. Determine who will be the key person or team members to remain up-to-date on drug shortages. Typically, a pharmacy purchasing agent is given this responsibility as he/she is often the first to know about a shortage. If the purchasing agent is not a pharmacist, a pharmacist should be assigned to work closely with the purchasing agent to aid in evaluating the clinical and operational impact of the shortage and to address means of handling the problem. Be alert to potential signs of an impending shortage, such as partially filled orders or specific strengths of drugs that are difficult to obtain. Regularly search the ASHP and FDA websites to learn about drug shortages. Social media, collegial networks with professional organizations, participation in professional listserves and discussion groups, wholesalers, and purchasing groups may also serve as resources about impending and new drug shortages.
Learn more about drug shortages. Once an impending or actual shortage has been identified, call the manufacturer for more details about the shortage, its estimated duration, and directions for ordering drugs on allocation or for emergent situations. Wholesalers and purchasing groups may also be a good source of information about drug shortages and alternatives.
Assess inventory of drugs on hand. Begin to assess the impact of the shortage by counting your inventory on hand and estimating how long the supply will cover your needs based on historical usage of the drug.
Research the drugs in short supply. Identify clinically appropriate uses of the drug, the lowest optimal dose for current indications, and strategies to decrease drug waste and inappropriate/unnecessary prescribing. Reference historical data from any drug use evaluation (DUE) conducted in the past or consider performing a DUE to determine how the drug is actually used in your facility.
Identify potential therapeutic alternatives early. Create and employ a standard, formal process for identifying and approving therapeutic alternatives to shortage drugs. An expedited approval process is also needed when the standard process is not timely enough to meet needs based on current inventory of the shortage drug. Obtain suggestions for therapeutic alternatives from the literature, professional websites, listserves, prescribers who use the product, and other local/regional hospitals (to promote consistency for prescribers who practice at multiple sites). Ensure that any decisions made about alternative drugs are in collaboration with medical, nursing, and pharmacy representatives, as well as any other disciplines that may use the product (e.g., respiratory therapists); involve a pharmacy and therapeutics committee as appropriate. Select alternatives early so an education plan can be developed in case implementation is needed. When appropriate, develop and approve guidelines for use of the alternative drugs. Also conduct an inventory of the current supply of approved therapeutic alternatives that will be used.
Prioritize patients and place limitations on use. Based on the extent of the shortage or shortage forecast, availability of alternatives, and results of DUEs, develop temporary therapeutic guidelines that reduce waste and tailor the drug’s use to groups of priority patients for whom the alternative drug may be unsafe, ineffective, or undesirable. Guidance on management of shortages and use limitations may be available from external organizations, such as government agencies (e.g., Centers for Disease Control and Prevention, departments of health), medical/professional organizations (e.g., Anesthesia Patient Safety Foundation), and specialty groups (e.g., American Society for Parenteral and Enteral Nutrition). Reassess how long the drug will be available to priority patients after conservation measures have been implemented. When appropriate, remove shortage drugs from unit floor stock and have pharmacy dispense the drugs as needed to better control use and waste.
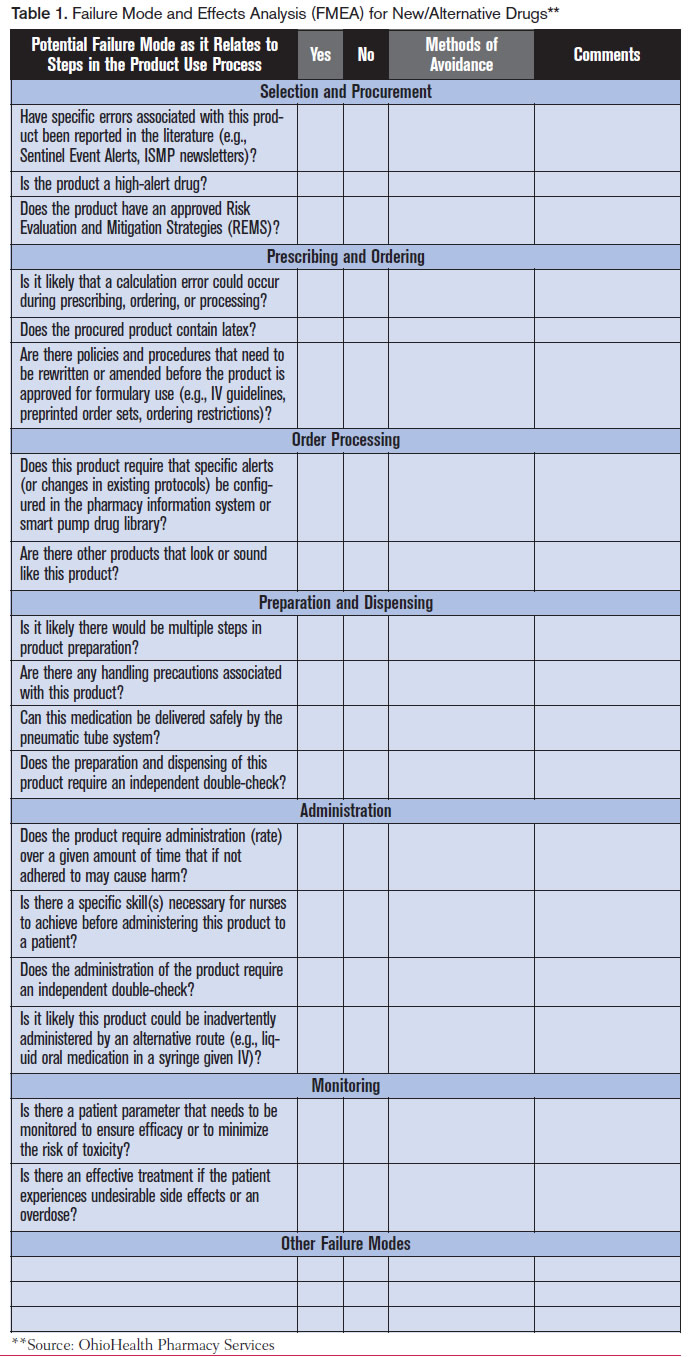
Conduct a failure analysis and take action. Considering the medical necessity of the shortage drug, the duration of the shortage, and the current supply of the drug, assess the potential hazard to patients and the organization. Conduct a mini failure mode and effects analysis (FMEA) to identify required changes to processes and potential misuses of alternative products. (A sample format for a mini FMEA from OhioHealth Pharmacy Services is available below) Determine how to best manage the risk of serious errors and adverse reactions to alternative drugs, and take action. Consider how the use of alternative drugs could affect current prescribing practices, storage of the drug, final product preparation (including directions for admixing), drug administration procedures, and the use of technology (e.g., electronic prescribing, bar-coding systems, automated dispensing cabinets, smart pump libraries). Make any necessary procedural and technological changes to support safe use of the alternative medications. Whenever possible, have pharmacy prepare and dispense alternative drugs in the most ready-to-use form. Also address any sound- and look-alike issues with an alternative drug’s name and packaging, and determine if additional safety checks, alerts, and/or patient monitoring are required when prescribing, preparing, dispensing, and administering an alternative product.
Do not hoard shortage or alternative drugs. Stockpiling a medication may lead to an artificial shortage where the drug might otherwise be available in adequate supplies to meet patient needs across the nation. Further, manufacturers with products in short supply rarely honor requests for quantities larger than historically ordered; allocations to organizations are typically determined by prior use.
Establish ongoing communication with staff. Using the most effective means possible (e.g., staff meetings, newsletters, email, website, Intranet, posters/charts, alerts in electronic systems), regularly share information with clinicians about:
- The drug shortage, causes, and expected duration (if known)
- Assessment of current drug availability
- Temporary therapeutic guidelines, including use limitations for the shortage drug
- Alternative products and how they will be supplied to units
- Dosing, preparation, and administration guidelines for alternative products
- Error potential with alternative products and how to reduce risk
- Additional patient monitoring and safety steps that may be required when using an alternative drug.
Consider preparing a report which includes the above information on the most critical drug shortages, updating the report on a daily basis, and using the report to keep healthcare professionals informed about the shortages (e.g., included in clinical staff email, staff meetings, the organization’s Intranet, newsletters). Pharmacy staff should be briefed daily regarding all aspects of drug shortages so they can serve as a resource to prescribers, nurses, and patients.
Engage ethics committee and risk management. When supplies of the shortage drug are critically low and suitable alternatives are not available or are suboptimal, consultation with an ethics committee may be required to help determine limitations on the use of the shortage drug. Potential or actual patient harm caused by the unavailability of a drug or a suitable alternative should be reported to risk management. (Organizations may want to engage the ethics committee and risk management staff in general terms to discuss potential ethical or risk management situations that may arise during a severe drug shortage, rather than waiting for a specific drug shortage to spur consultation.)
Establish a drug shortage network with other local healthcare providers. Build and strengthen local collaborative networks to share information regarding drug shortages and alternative products, to share emergency supplies of shortage drugs when appropriate, and to coordinate the transfer of patients to providers that still have a shortage drug available when an alternative is not suitable.
Determine an organizational position on alternative suppliers. The potential availability of shortage drugs from secondary gray markets, compounding pharmacies, or ramped up in-house compounding are complex issues that require philosophical discussions as well as quality and budgetary considerations to determine whether it is appropriate and safe to utilize these resources. Decisions regarding these issues are difficult to make under pressure, so the organizational position should be well known and documented. Requirements regarding quality control measures for outside vendors and criteria for when the current position might be challenged should be included.
Proactively monitor adverse events associated with drug shortages. Utilize error and adverse event reporting systems as well as a hotline, chart review, focus group meetings, discussions during pharmacy rounds, or other means to learn about hazardous conditions, near misses, and adverse events associated with drug shortages so actions can be taken to limit further risk and harm.
As a final recommendation, healthcare organizations might want to share the results of our recent survey on drug shortages with pharmacy and therapeutics committee members and key groups of organizational leaders and clinicians, including nurses and the medical staff, to help illustrate the significant impact of drug shortages, particularly the types of adverse events that are happening across the nation. The full results of our survey can be found in our September 23, 2010, newsletter.
References:
- ISMP. Part II of our national survey on drug shortages: proactive guidelines to safely managing scarce supplies. ISMP Medication Safety Alert! April 4, 2001;6(7):1-2.
- Fox RE, Birt A, Janes KB, Kokko H, et al. ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health-Syst Pharm. 2009;66:1399-1406.
